Draw Resonance Structures for Each of the Following
Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn. 30 marks For each of the following molecules.

Resonance Structures 4 Rules On How To Evaluate Them With Practice Organic Chemistry Chemistry Help Chemistry Worksheets
In step 1 draw the resonance form that forms a carbocation next to the oxygen.
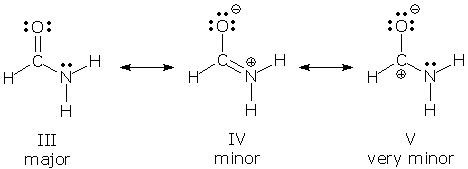
. In step 1 draw the resonance form that forms a secondary carbocation. This general chemistry video tutorial provides a basic introduction into resonance structures. Show the electron shift using curved arrow.
Draw resonance structures for the following compound. We review their content and use your feedback to keep the quality high. It explains how to draw the resonance structures using curved.
Click hereto get an answer to your question Draw resonance structures for each of the following compounds. Find step-by-step Chemistry solutions and your answer to the following textbook question. It gives the following structure and has a multiple bonds and an adjacent atom with one lone pair of electrons.
Draw your resonance structures so that the atoms in them are bonded together in this order. The more stable structure is O C N-. 100 3 ratings Transcribed image text.
Sulfur dioxide SO 2 molecule resonance structures. All the charges are as shown but the lone pairs of electrons might be omitted so add the lone pairs to help you track the movement of electrons. If by that you mean you want N to have a formal charge of 1- then draw it as O C N-with 2 lone pairs of electrons around both the N and O atom.
The most stable resonating structure of C H 3. This species has its three atoms bonded sequentially in the following fashion. Step 2 Get help answering Molecular Drawing questions.
11 Choose draw the structure that will easily react with the NaOH baseThat is the structure that will be more reactive_ b_ When your chosen structure in a above reacts. Draw resonance structures for each of the following compounds and make sure to also draw curved arrows that show how your resonance structures were generated. 2 marks each state the 3-dimensional structure predicted by VSEPR and iii.
List in them order from highest to lowest frequency. The more resonating structures a molecule or ion has the more stable it becomes. Mathrm SO _ 2.
In step 1 draw the resonance form that forms a secondary. X Your answer is incorrect. We can use the same procedure as outlined above to obtain the Lewis structure.
Problem 54 Medium Difficulty. Resonating structures represent the movement of electron clouds inside a molecule when it has alternating double bonds. Both would represent resonance structures however which is what the question asked for.
For each of the following draw reasonable resonance structures. You can see first two structures have charges on atoms. We can draw three resonance structures for CO 3 2-ion as above.
Include lone pairs and charges in your structure. Lets take two more examples to learn how to draw resonance structures. Draw resonance structures for each of the following compounds.
Chemistry questions and answers. Solve Study Textbooks Guides. Experts are tested by Chegg as specialists in their subject area.
3 marks each predict the bond orders for each type of bond present. Add curved arrows to show the resonance. MathrmClNO_2mathrmN is the central atom b.
We can draw three resonance structures for SO 2 molecule. O_2 b. Include lone pairs and charges in your structure.
Draw the resonating structures of NO 3 CH 3 COOCH 2 CHCH 2 SO 32. Nitrogen dioxide NO 2 molecule. Your answer is correct.
7 Use the structures shown below. Draw Lewis structures for each of the following molecules. Join Login Class 11 Chemistry Organic Chemistry - Some Basic Principles and Techniques.
For each of the following compounds draw the resonance structures. Draw resonance structures for each of the following compounds. IMAGE IS NOT AVAILABLE TO COPY.
N_2 c. 0 Edit CH CH2 H2C HC on of lohn Wiley Sons IndVersion 424. Highly contributing a O3 b CH3CNO c CH3NCO e CH3NCS d CH3SCN O O O H3 C N O H3C C N O H3C N C O H3C N C O H3C S C N H3C S C N H3C N C S H3C N C S highly contributing highly contributing H3C C N O-2 very low contributing resonance.
So we can understand structure three is more stable than other two structure. Practice on Resonance Structures - Answers 1. Write contributing important resonance structures for each of the following compounds and predict their relative CO vibrational frequencies based on the importance of the contributing resonance structures.
This IS NOT the most stable form of OCN-however. Resonance is an exercise within a molecule following the Valence. MathrmOCN-mathrmC is the central atom.
102 Draw resonance structures for. Previous question Next question. Using curved arrows draw at least one resonance structure for each of the following species.
5 marks each draw Lewis structures showing resonance structures and formal charges where applicable ii. Draw resonance structures for each of the following molecules or ions. To answer the following questions.
Show resonance structures if they exist. But in third structure there are no charges on atoms.
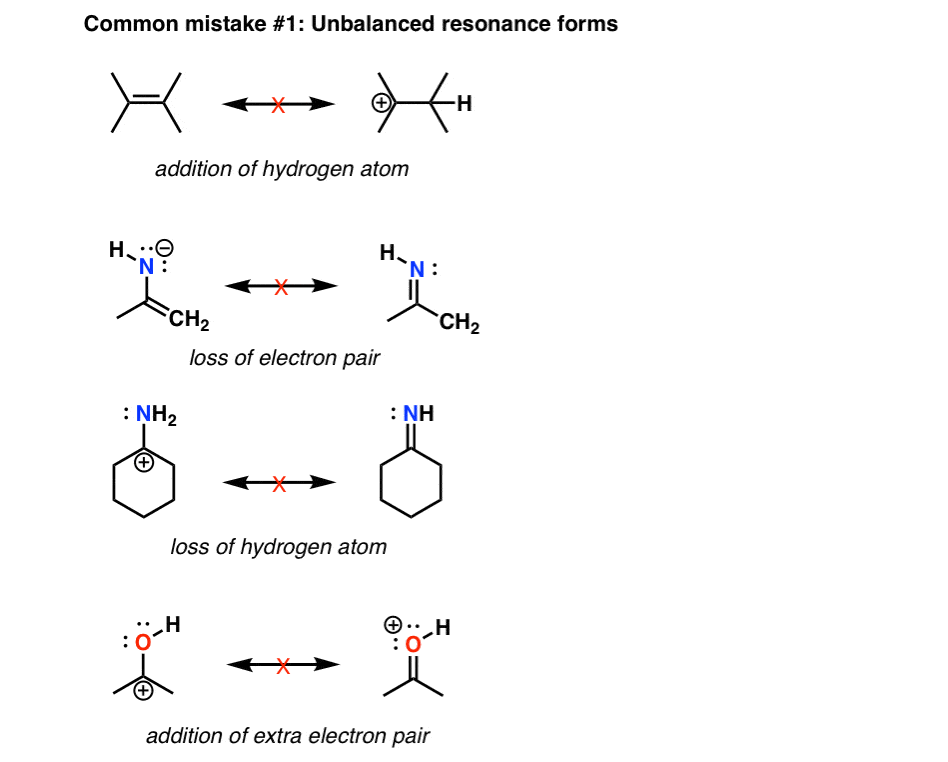
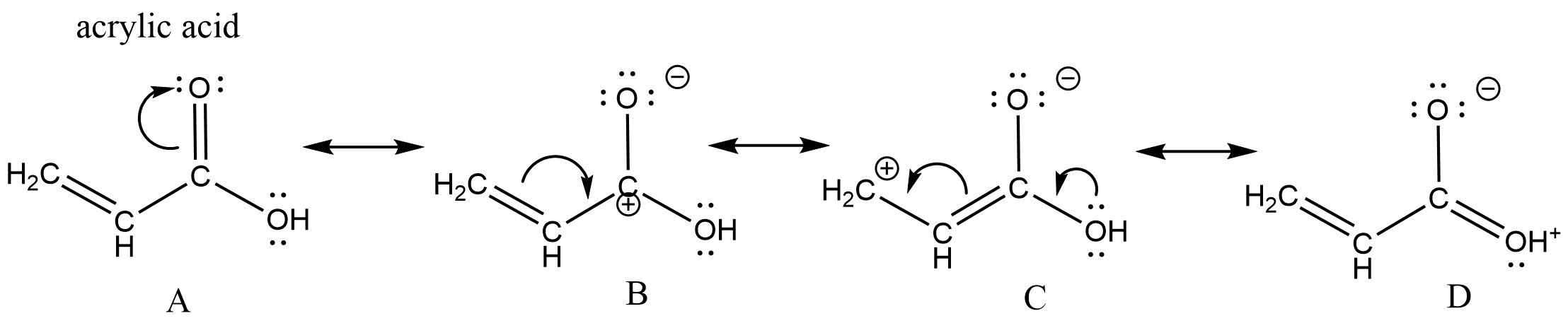
No comments for "Draw Resonance Structures for Each of the Following"
Post a Comment